Structural Nature
Collagen is a major structural component of skin tissue. It is the most abundant fibrous protein in higher vertebrates because it is the principal constituent of skin and connective tissue as well as being present in bones and teeth (Milano). More than a third of the proteins synthesized by the living cells of the human body consists of collagens and collagen Type I is the majority structural protein (Micutz et al).
This tough, elastic, and versatile structural protein is made up of three polypeptide chains arranged in a triple helix. Type I collagen has two a1(1) and one a2(1) chain composition where the a1(1) and the a2(1) chains are homologous (Gelse et al). Cells assemble Type I collagen proteins from the smaller amino acids-proline, glycine, and hydroxyproline. Proline and glycine are available through dietary sources. Hydroxyproline is found almost exclusively in collagen itself. Specific amino acids create specific functions for collagen. The individual triple helices are arranged to form fibrils that are of high tensile strength and flexibility and can be further assembled and cross-linked to support stress efficiently (Chvapile et al). Collagen is a predominant component of the extracellular matrix (ECM) that provides both strength and support, in addition to assisting in tissue formation and maintenance. Type I collagen is the main structural protein, defined by giving high mechanical strength to tissues; having good biocompatibility, low antigenicity and ability to be crosslinked; and tailored for its mechanical, degradation and fluid-uptake properties (Malafaya et al)…
Bioactive Nature
Collagen is highly involved in cell signaling. ECM proteins have been found to possess cryptic peptide residues in collagen molecules which come into play after biodegradation or biosorption of the parent molecule. The proteolytic hydrolysis of the ECM protein releases the cryptic peptides with many novel biological activities not exhibited directly by the parental protein which include angiogenic, antimicrobial, mitogenic, and chemotactic properties (Banerjee et al). Its importance as a biomaterial and long history of use in medical and wound healing applications is due to its biodegradable and biocompatible properties and low immunogenic effect. Collagen plays a critical role in tissue development and regeneration by supporting the proliferation and differentiation of cells. Collagen remodeling involves both collagen degradation by proteases and synthesis (Yang et al).
Wound Healing Nature
Medical applications for collagen include use as an absorbable hemostatic agent. Collagen is well known to participate in the intrinsic activation of the blood coagulation process and in activating platelets (Gabay et al); is commonly used as a wound-healing agent that stimulates tissue growth; is used as a vehicle in surgical prosthesis and surgical closure and as a material for implantation. Collagen is a crucial component of the extracellular matrix (ECM) during wound healing or tissue remodeling processes. Collagen is the most abundant of the many substances released into the ECM upon stimulation via a wound or ulceration. During wound healing, a complex series of events involving collagen occurs. Collagen is a stimulating agent through its interaction with various growth factors to capture fibronectin, as well as the migration and replication of cells.
Within the initial inflammatory phase, collagen assists with homeostasis to stop blood loss; attracts macrophages to the region as a chemoattractant to fight infection and induce collagen synthesis; and enhances inflammatory infiltration to improve natural wound cleansing (Chvapil et al). Collagen attracts fibroblasts and keratinocytes to the wound and is the key protein in establishing a scaffold for the healing process as well as bridging vascular basement membranes for angiogenesis in the ECM. Angiogenesis leads to the delivery of cells, platelets, and macrophages to the region to protect the wound from infection via inflammatory reactions (Chung & Ferrara).
During the proliferative phase, collagen acts as a scaffold for fibroblast attachment to initiate collagen synthesis. As granulation tissue develops, additional fibroblasts are attracted to the wound site. Angiogenesis results in the development of a scaffold of collagen fibers and becomes a template for new tissue growth. The scaffold accelerates the healing response and chemotactically absorbs the fibroblasts, preserves them, and facilitates their proliferation to induce proper healing.
During the maturation phase, collagen enhances the deposition of oriented, organized collagen fibers as the connective tissue matric reoraganizes. Collagen fibrils consolidate into thicker fibers, imparting strength to the tissue over time to replace and reinforce weaker tissue. The result is cells gaining more tensile strength (Brenner). Collagen is apparent in every stage of the healing process to support natural cell interactions such as proliferation and migration and is an essential resource in healing wounds (Rather).
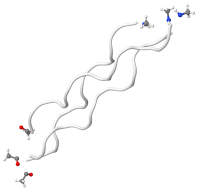
Denatured/hydrolyzed collagen structure
Hydrolyzed collagen is produced by boiling water to break down hides and bones; altering the molecular structure of the collagen proteins through a process called denaturing. Heat or chemicals can also be used to denature collagen but destroys more nutrients. The final stage of processing involves reconstituting the collagen. Hydrolyzed collagen is commonly used as an oral supplement to support general skin and nail growth.
When used in wound dressings, modified or denatured collagen often uses fillers such as calcium alginate to improve absorption rates (Brenner et al). Clinicians must be careful to flush out the wound at each dressing change to remove the alginate or cellulose fillers that may contribute to bacterial growth and/or stall the healing process.
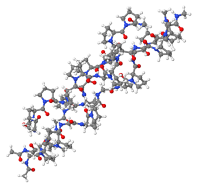
Non-hydrolyzed collagen structure
Native, intact collagen produced with little or no heat and limited processing provides a natural long-chain scaffold or substrate for new tissue growth (Hochstein et al). The manufacturing process ensures the collagen remains biologically active in its native, triple helix form and its immunomodulating ability intact. It is important that collagen fibers retain their native structure. For this reason, the method of isolation and purification of collagen fiber from the starting material should extract all noncollagenous components of the original tissue without denaturing the original crystalline structure of collagen (Barkin). When purified properly, native intact collagen provides a natural scaffold for new tissue growth, is biocompatible and plays a critical role in tissue development and regeneration by acting as a temporary scaffold to provide the wound with an alternative collagen source that can be degraded by high levels of MMPs as a sacrificial substrate for tissue regeneration to support proliferation and differentiation of cells (Hochstein et al).
HELIX3® Bioactive Collagen is a 100% Type I bovine collagen that retains its full triple helix collagen structure as a non-denatured collagen dressing. Due to this retention of structure —not demonstrated in all collagen products—there is significantly more (40-60x) absorption by weight of collagen into damaged tissue to attract more macrophages and fibroblasts during the inflammatory and proliferation of healing. When activated, HELIX3 has a pH of 3, providing a very acidic and harsh environment for bacterial growth. Moreover, its unique size and structure leads to greater absorption of wound fluids, naturally reducing the risk of infection.
- Banerjee P, Shanthi: Protein & Peptide Letters: 2016:23 (7)
- Barkin; Method of making collagen fibers for surgical use. US Pat. No 3,374,103
- Brenner M., Pradeep, A., Raminfard A.; Collagen Treatment in the Diabetic Foot. Podiatry Management, Dec 2015: 111-118.
- Brenner MA, Tracking the Diabetic Foot: Adjunctive Treatment with Collagen Material. Advances in Wound Care 1994: 7(6): 44-52.
- Chung A., Ferrara N. The Extracellular Matrix and Angiogenesis: The Role of the Extracellular Matrix in Developing Vessels and Tumor Angiogenesis., Pathways. 2010: 11:1-5.
- Chvapil M, Koronemthal RL, Van Winkle W. Medical and Surgical Applications of Collagen.
- Chvapil M., Chvapil, B.S., Owen, J.A.; Comparative study Study of Four Wound Dressings on Epithelialization of Partial-Thickness Wounds in Pigs. J Trauma 1987: 27:278-282.
- Gabay M., Absorbable Hemostatic Agents: Am J Health System Pharm. 2006:63 (13)” 1244-1253).
- Gelse K, Poschl E, Aigner T; Collagens-structure, function, and biosynthesis. Science Direct. Drug Delivery Reviews 55 (2003) 1531-1546.
- Hochstein A.O., Bhatia, A; Collagen: It’s Role in Wound Healing. Podiatry Management. Aug 2014. 103-110.
- Malafaya PB, Silva GA, Reis RL. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv Drug Deliv Rev. 2007: 59:207-233. Doi: 10.1016/j.addr.2007.03.012. Bio Drug.
- Micutz M, Staicu Tm, Sulea D, Leca M, Ghica C. Absorption complexes of collagenous polypeptideionic surfactant in aqueous medium. Revue Roumaine de Chimie. 2010: 55(8) 501-10.
- Milano, Euroresearch S.R. I., Patent Application.
- Raher E. Collagen and the Phases of Wound Healing. Wound Caring 1998: 5:2.
- Yang W, Chan VC, Kirkpatric A, Ramshaw JAM, Brodsky B. Prediction of collagen stability from amino acid sequence. J. Biol, Chem 2005: 280:19343-19349 (PubMed: 11805290).
AMERX Health Care Corp.
Founded in 1993 by John C. Anderson, AMERX Health Care Corporation is dedicated to providing superior wound and skin care products that deliver positive outcomes, increase patient compliance, and reduce treatment costs. Long-known for the top-rated AMERIGEL® line of topical wound and skin care products featuring Oakin® (oak extract), AMERX Health Care has expanded our offerings throughout the years. We now offer a comprehensive suite of wound care products including HELIX3® Bioactive Collagen, AMERX® Kits & Dressings, and the innovative EXTREMIT-EASE® Compression Garment.